Have you heard of the revolutionary Watchman Device, which is creating a buzz in medical circles? We’ll dive into the Watchman device controversy and distinguish the real facts.
The Watchman Device Controversy
The use of the Watchman Device, a novel device to reduce stroke risk in patients with atrial fibrillation (AF), has been controversial. This small, implantable device has the potential to revolutionize stroke prevention, but how it got there is anything but conventional.
The questions surrounding it revolve around its safety and efficacy as well as how long-term the effects can be. Healthcare sites are questioning whether it is just as reliable and safe as existing blood thinners. Critics say its long-term health and quality-of-life effects have been under-tested.
Moreover, supporters say it’s a game-changer for patients with an intolerance to long-term blood-thinning medications. They focus on how it could reduce the risk of strokes without life-long drugs, which they say would improve quality of life and relieve health care burdens.
Both doctors and patients will need to continue digesting the data as debates around the advantages of the Watchman Device compared to traditional treatments heat up. The medical community keeps looking at the best protections against strokes.
How the Watchman Device Works
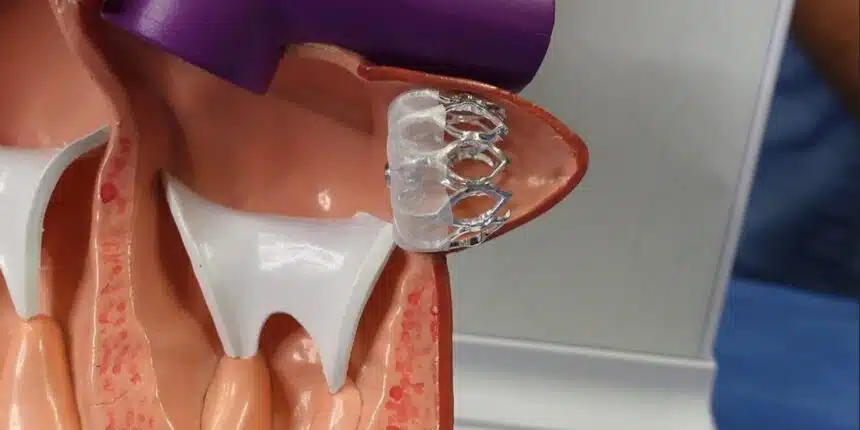
The Watchman Device is a small implant that goes into the part of the heart called the left atrial appendage (LAA), where clots in people with AF are most often formed, leading to increased stroke risk.
This device prevents clots from traveling to the brain by blocking the opening to the LAA, where more than 90 percent of clots form in patients with atrial fibrillation. Constructed of a flexible nickel-titanium frame and porous fabric, it conforms to the LAA, forming a barrier against clots.
This is done under anesthesia by cardiologists or cardiac surgeons. The procedure is performed minimally invasively through a small groin incision, where a catheter is used to deliver the device into the heart. The process is typically brief, and patients often return home the same day or the next day.
Following implantation, patients will temporarily take blood thinners (45 days to 6 months) while the device is assimilated into the heart tissue. Subsequently, many of those patients stop taking blood thinners, and they’re left to depend on the device for stroke protection.
Understanding the Risks and Side Effects of the Watchman Device
The Watchman Device provides another option for patients with atrial fibrillation who are at high risk for stroke. Still, the procedure is not without risks and side effects.
Potential complications include bleeding, the formation of a blood clot, and the device moving to another part of the body (device embolization). These problems can be severe and require further medical attention or an operation.
There is also a danger of device-related infections. They can also occur if the device becomes contaminated during implantation or doesn’t integrate properly with heart tissue. The infections can cause serious problems, such as endocarditis, an infection of the heart lining.
Then there is the long-term reliability of the device that’s still up in the air. It should be permanent, but there is a risk that it could break or not hold, and if so, you may need more procedures to fix or remove it.
While these risks are not insignificant, the Watchman Device has successfully completed extensive clinical trials with a much better safety profile than alternate treatments. The FDA-approved it, meaning the benefits typically outweigh the risks for appropriate patients.
Still, all patients considering the Watchman Device should discuss the pros and cons with their healthcare providers.
Patient Experiences with the Device
Now that the Watchman Device has been around for a long time, patients are sharing their stories and experiences, regardless of the clinical trials results.
Most of the patients find great relief after the Watchman Device. Those who have fought blood thinning medications will be the first to appreciate being free from daily pills and a never-ending cycle of health checks.
Some have also observed a decrease in stroke prevention—and treatment-related costs. This is especially useful to the uninsured or insured by high-deductible plans, and hence, Watchman Device was a very cost-effective option for them.
But not every experience is a good one. Many reported complications such as bleeding or clots that require additional medical intervention (including implant removal). These raise questions about the safety and durability of the device.
Nonetheless, most patients are satisfied after the procedure and experience an improvement in device performance. They are more in control of their atrial fibrillation and not as afraid of stroke risk, which is a great treatment option.
Has anyone died from the Watchman procedure?
Rarely, the Watchman procedure itself may cause an air embolism. This is because surgeons insert the Watchman through the veins. There is evidence that at least one person has died due to an air embolism related to a Watchman procedure. Five out of 449 Watchman procedures can result in an air embolism-related stroke.
Final Thoughts on the Watchman Device Controversy
The Watchman Device is a major advance for stroke prevention as of now, aside from drugs. The Watchman system is an option for people who want to get away from long-term medication by blocking off the left atrial appendage, which could help reduce stroke risk and improve their quality of life. Still much of the medical community remains polarized over safety and efficacy issues. Further study is crucial to determine the Watchman Device’s role in care.
Before you go, you may want to read more about Autism Speaks Controversy. Also, follow us on Facebook and Twitter to stay informed about the latest buzz and exciting stories.